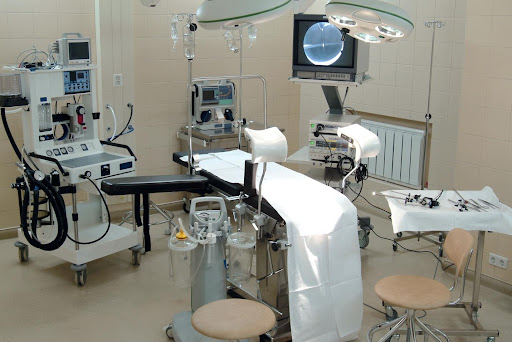
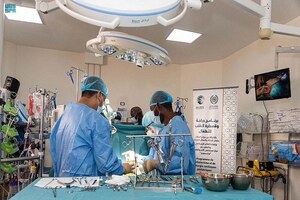
The County Government of Nyeri intends to invest at least Sh1.6 billion in various development projects spread across the county’s eight sub-counties.
The funds expected to be spent over the remainder of the fiscal year 2023/2024 will be used to improve key infrastructure projects such as markets and basic social utility facilities such as hospital completion.
Governor Mutahi Kahiga said in his New Year’s address to Nyeri residents that his administration will spend a significant amount of money renovating milk processing plants and upgrading rural roads to improve agricultural product transit from farms to markets.
“From our county-specific projects, we will spend Sh1.6B in development. These range from the county industrial park, the completion of Karatina Level Hospital emergency block, various renovation works in all our hospitals and equipping sanitation, among many other projects,” said his statement.
“We have operationalized the Field Marshal Muthoni Kirima Bus Park, which should be fully occupied by the end of January. The Narumoru hospital has taken off albeit with staffing challenges. We will recruit 125 medical personnel to further boost the staffing. This will allow all disputes to be resolved,” he stated.
According to figures from the Office of the Chief Officer, Economic Planning and Budgeting, the county spent a total of Sh89,248,853 during the first quarter of the fiscal year 2023/2024 to fund various development projects in the county.
Kahiga stated that a total of Sh735 million had been set up to fund various development projects across the 300 wards to demonstrate the cordial working relationship between the executive and members of the County Assembly.
“Our County Assembly was rated the best in Kenya, thanks to the honourable members. This year, the Honourable Ward Representatives have lined up 300 ward projects to cost Sh735,000,000. These projects cover all departments and range from roads, streetlights, hospitals, dispensaries, bridges and agricultural programmes,” stated Mutahi Kahiga.
The Governor also stated that the county had cleared all pending bills owed to suppliers, praising his administration’s fiscal discipline.


 “The 24th MEDEXPO KENYA 2024” has been announced from the 17 – 19 April 2024. The longest running premier trade exhibition will showcase the widest range of products and equipment for the medical, pharmaceutical and healthcare sector. The trade fair, will be held at the Sarit Expo Center in Nairobi and will attract the interest of decision makers and trade representatives from not just Kenya and the East African Region but the entire African continent.
“The 24th MEDEXPO KENYA 2024” has been announced from the 17 – 19 April 2024. The longest running premier trade exhibition will showcase the widest range of products and equipment for the medical, pharmaceutical and healthcare sector. The trade fair, will be held at the Sarit Expo Center in Nairobi and will attract the interest of decision makers and trade representatives from not just Kenya and the East African Region but the entire African continent.