RIYADH: In Kenya and Cameroon, the King Salman Humanitarian Aid and Relief Centre (KSrelief) of Saudi Arabia launched two volunteer medical programmes.
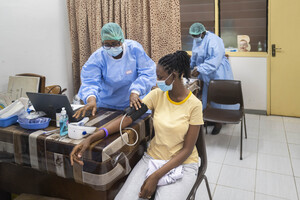
13 volunteer doctors from the medical staff of the charity organisation operated on patients in Nairobi who needed cardiac catheterizations. According to the Saudi Press Agency (SPA), the programme, which ran from May 22 to May 26, was implemented in conjunction with the Al-Balsam International Organisation.
The project is an expansion of the voluntary medical programmes the KSrelief currently has in place in across a variety of topics to assist people and families in need in developing countries, according to a statement from the SPA.
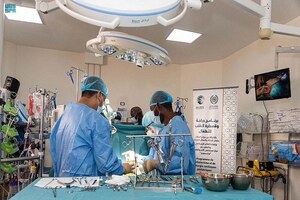
In the city of Maroua in Cameroon, 22 volunteer surgeons from a variety of specialties, including orthopaedics, paediatrics, gynaecology, and obstetrics, carried out medical procedures.
Additionally, KSrelief kept running its medical programmes in other parts of the globe
Al-Jadah Health Centre clinics in Yemen’s Hajjah governorate treated 7,618 patients in April with the assistance of KSrelief. Through its several clinics for maternity, radiography, surgery, paediatrics, internal medicine, and reproductive health, the centre provided healthcare.
The Subul Al-Salam Social Association’s ambulance service in Al-Minya, Lebanon, received funding from KSrelief and completed 90 missions in one week. The tasks included anything from taking patients to and from hospitals to giving first assistance.
The global food aid programmes were still being carried out by the Saudi aid organisation. 750 earthquake-affected families in Idlib, Syria, got food shipments and hygiene kits.
The group also sent 25 tonnes of dates to Burundi as a gift from the Kingdom.